- BioVector NTCC典型培养物保藏中心
- 联系人:Dr.Xu, Biovector NTCC Inc.
电话:400-800-2947 工作微信:1843439339 (QQ同号)
邮件:Biovector@163.com
手机:18901268599
地址:北京
- 已注册
Helga Kolb
Three basic types of glial cell are found in the human retina, Muller cells, astroglia and microglia. All were described for the retina by Cajal more than one hundred years ago (1892).
1. Muller cells.
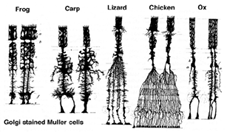
Muller cells are the principal glial cell of the retina. They form architectural support structures stretching radially across the thickness of the retina and are the limits of the retina at the outer and inner limiting membrane respectively. A complete understanding of the shape of a Muller cell is best seen after Golgi staining as shown originally by Cajal (1892) below.
Fig. 1. Golgi stained Muller cells
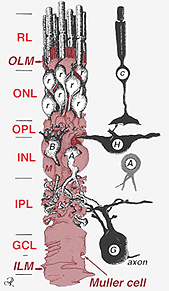
Muller cell bodies sit in the inner nuclear layer and project irregularly thick and thin processes in either direction to the outer limiting membrane and to the inner limiting membrane. Muller cell processes insinuate themselves between cell bodies of the neurons in the nuclear layers and envelope groups of neural processes in the plexiform layers (Fig. 1). In fact retinal neural processes are only allowed direct contact, without enveloping Muller cell processes, at their synapses.
A single progenitor cell gives rise to both Muller cells and retinal neurons(Turner and Cepko, 1987) although apparently in two phases. The earliest phase neurons born at the apical margin of the neuroepithelium adjacent to the pigment epithelium produces primary neurons consisting of cone cells, horizontal cells and ganglion cells (Fig. 2, right). The second phase of cells also born at the apical margins produces Muller cells and rod photoreceptors, bipolar cells and amacrine cells (Reichenbach and Robinson, 1995) (Fig. 2, left). All the developing neurons and the Muller cells have to migrate inward to their final position and it is thought that the Muller cell processes and trunks guide much of the neuron migrations and direct the neurite differentiations.
Fig. 2. 3-D schematic drawing of the relationship between Muller cell and other retinal neurons
The junctions forming the outer limiting membrane are between Muller cells and other Muller cells and photoreceptor cells as sturdy desmosomes or zonula adherens. In some species gap junctions (specialized membrane associations and channels that allow passage of small molecules and ions) or tight junctions are part of these Muller cell junctions (Miller and Dowling, 1970) but not so in mammalian species where no dye coupling has ever been observed (Robinson et al., 1993; Reichenbach and Robinson, 1995). The surface of the Muller cell facing the pigment epithelium and subretinal space is expanded by many projections of the Muller cell membrane known as apical villi. The inner limiting membrane, on the other hand, is formed by the conical endfeet of the Muller cell but no specialized junctions are seen here. Muller cells also form endfeet on the large retinal blood vessels at the inner surface of the retina. The surface of the Muller cell membrane facing the vitreous is covered with a mucopolysaccharide material and thus forms a true basement membrane.
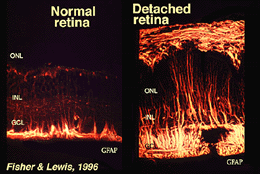
Muller cells contain glycogen, mitochondria and intermediate filaments which are immunoreative for vimentin and to some extent to glial fibrillary acidicprotein (GFAP). The latter filaments are normally in the inner half of the retinal Muller cells and their endfeet (Fig. 3, left), but following trauma to the retina such as retinal detachment, both vimentin and GFAP are massively upregulated and found throughout the cell (Fig. 3, right) (Guerin et al., 1990; Fisher and Lewis, 1995).
Fig. 3. GFAP immunoreactivity in Muller cells
Muller cells have a range of functions all of which are vital to the health of the retinal neurons. Muller cells function in a symbiotic relationship with the neurons (for an excellent review see Reichenbach and Robinson, 1995). Thus Muller cell functions include:
Supplying endproducts of anaerobic metabolism (breakdown of glycogen) to fuel aerobic metabolism in the nerve cells.
They mop up neural waste products such as carbon dioxide and ammonia and recycle spent amino acid transmitters.
They protect neurons from exposure to excess neurotransmitters such as glutamate using well developed uptake mechanisms to recycle this transmitter. They are particularly characterized by the presence of high concentrations of glutamine synthase.
They may be involved in both phagocytosis of neuronal debris and release of neuroactive substances such as GABA, taurine and dopamine.
They are thought to synthesize retinoic acid from retinol (retinoic acid is known to be important in in the development of the eye and the nervous system) (Edwards, 1994)
They control homeostasis and protect neurons from deleterious changes in their ionic environment by taking up extracellular K+ and redistributing it.
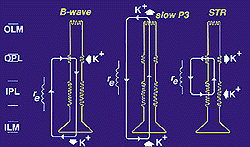
They contribute to the generation of the electroretinogram (ERG) b-wave (Miller and Dowling, 1970; Newman and Odette, 1984), the slow P3 component of the ERG (Karwoski and Proenza, 1977) and the scotopic threshold response (STR) (Frishman and Steinberg, 1989). They do so by regulation of K+ distribution across the retinal vitreous border, across the whole retina and locally in the inner plexiform layer of the retina (Fig. 4, from Reichenbach and Robinson, 1995, adapted from Newman, 1989).
Fig. 4. Regulation of K+ by Muller cells in Muller cells
2. Astrocytes.
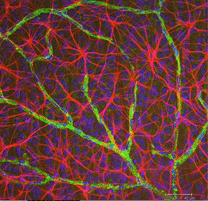
Astrocytes are not glial cells of the retinal neuroepithelium but enter the developing retina from the brain along the developing optic nerve (Stone and Dreher, 1987; Chan-Ling 1994). They have a characterisic morphology of a flattened cell body and a fibrous series of radiating processes. Intermediate filaments fill their processes and thus they stain dramatically with antibodies against GFAP (Schnitzer, 1988). Astrocyte cell bodies and processes are almost entirely restricted to the nerve fiber layer of the retina. Their morphology changes from the periphery to the optic nerve head: from a symmetrical stellate form in peripheral retina (Figs. 5a and b) (Schitzer, 1988) to extremely elongated near the optic nerve (Fig. 6 and 7).
In immunocytochemical staining (Fig. 5b) and in HRP intracellular injections (Fig. 7) stained astrocytes clearly exhibit processes aligned along the ganglion cell axons coursing through the nerve fibre layer. In distribution, astrocytes reach their peak on the optic nerve head and have a fairly uniform decline in density in radiating rings from the nerve head. They are not present in the avascular fovea or ora serrata.
 Fig. 6. Astrocytes in central retina. Schnitzer, 1988 | 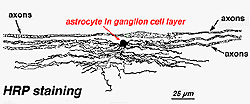 Fig. 7. Astrocytes in cat retina stained by intracellular injection of HRP. Courtesy of Ralph Nelson. |
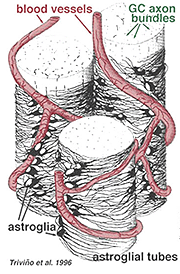
Thick and thin astrocytes have been distinguished on the basis of staining with antibodies to GFAP (Trevino et al., 1996). Thus astrocytes are arranged over the surface of the ganglion cell axon bundles as they course into the optic nerve head forming a tube through which the axons run (Fig. 8). Gap junctions and zonula adherens junctions have been described between astrocytic processes in cat retina (Höllander et al., 1991).
Fig. 8. 3-D block of astrocytes arranged over the surface of ganglion cell axon bundles
The blood vessels running in and among the ganglion cell bundles are also covered by by both processes and even an occasional cell body of an astrocyte. The function of astrocytes enveloping ganglion cell axons and the relationship to blood vessels of the nerve fibre layer suggests they are axonal and vascular glial sheaths and part of a blood-brain barrier. Similar to Muller cells, they are known to contain abundant glycogen and they may form a nutritive service in providing glucose to the neurons. In addition they probably serve a role in ionic homeostasis in regulating extracellular potassium levels and metabolism of neurotransmitters like GABA.
3. Microglial cells.
The third glial cell type is supposedly of mesodermal origin and thus, strictly speaking are not neuroglial as are the astrocytes and Muller cells. They enter the retina coincident with the mesenchymal precursors of retinal blood vessels in development (Chan-Ling, 1994). Microglial cells are ubiquitous in the human retina being found in every layer of the retina.
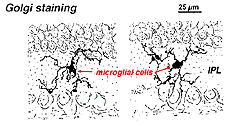
In Golgi-stained retina they look like strange, multipolar forms with small cell bodies and irregular short processes. In fact, in Golgi preparations they have sometimes been mistaken for nerve cells particularly when they lie in a nuclear layer with a single orientation of their processes in the plexiform layer.
Fig. 9. Golgi staining of microglial cells
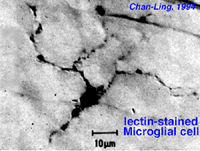
Microglial cells may be of two types. One form is thought to enter the retina at early stages of development from the optic nerve mesenchyme and lie dormant in the retinal layers for much of the life of the retina. The other form of microglia appear to be blood-borne cells, possible originating from vessel pericytes (Boycott and Hopkins, 1981; Gallego, 1986). Both types can be stimulated into a macrophagic function after trauma to the retina, and then they engage in phagocytosis of degenerating retinal neurons.
Fig. 10a. Lectin-stained microglial cell from Chan-Lin, 1994.
4. References.
Boycott BB, Hopkins JM. Microglia in the retina of monkey and other mammals; its distinction from other types of glia and horizontal cells. Neuroscience.1981;6:679–688. [PubMed]
Cajal SR. In: Thorpe SA, Glickstein M, translators. 1892. The structure of the retina. Springfield (IL): Thomas; 1972.
Chan-Ling T. Glial, neuronal and vascular interactions in the mammalian retina. Prog. Ret. Eye Res. 1994;13:357–389.
Edwards RB. Biosynthesis of retinoic acid by Müller glial cells: a model for the central nervous system? Prog. Ret. Eye Res. 1994;13:231–242.
Fisher SK, Lewis GP. Photoreceptors and beyond: cellular and molecular effects of retinal detachment.2nd Great Basin Visual Science Symposium, II, University of Utah Press. 1995
Frishman LJ, Steinberg RH. Light-evoked increases in [K+]o in proximal portion of the dark-adapted cat retina. J Neurophysiol. 1989;61:1233–1243. [PubMed]
Gallego A. Comparative studies on horizontal cells and a note on microglial cells. Prog. Ret. Res. 1986;5:165–206.
Guerin CJ, Anderson DH, Fisher SK. Changes in intermediate filament immunolabeling occur in response to retinal detachment and reattachment in primates.Invest. Ophthal. Vis. Sci. 1990;31:1474–1482. [PubMed]
Karwoski CJ, Proenza LM. Relationship between Muller cell responses, a local transretinal potential, and potassium flux. J Neurophysiol. 1977;40:244–259.[PubMed]
Miller RF, Dowling JE. Intracellular responses of the Muller (glial) cells of the mudpuppy retina: their relation to b-wave of the electroretinogram. J Neurophysiol.1970;33:323–341. [PubMed]
Newman EA, Odette LL. Model of electroretinogram b-wave generation: a test of the K+ hypothesis. J Neurophysiol. 1984;51:164–182. [PubMed]
Newman EA. Electrophysiology of retinal glial cells. Prog. Ret. Res. 1989;8:153–172.
Reichenbach A, Robinson SR. The involvement of Müller cells in the outer retina. In: Djamgoz MBA, Archer SN, Vallerga S, editors. Neurobiology and clinical aspects of the outer retina. London: Chapman & Hall; 1995. p. 395-416.
Robinson SR, Hampson ECGM, Munro MN, Vaney DI. Unidirectional coupling of gap junctions between neuroglia. Proc. Austr. Neurosci. Soc. 1993;3:167.
Schnitzer J. Astrocytes in mammalian retina. Prog. Ret. Res. 1988;7:209–232.
Stone J, Makarov F, Hollander H. The glial ensheathment of the soma and axon hillock of retinal ganglion cells. Vis Neurosci. 12:273–279. [PubMed]
Stone J, Dreher Z. Relationship between astrocytes, ganglion cells and vasculature of the retina. J Comp Neurol. 1987;255:35–49. [PubMed]
Trivino A, Ramirez JM, Salazar JJ, Ramirez AI, Garcia-Sanchez J. Immunohistochemical study of human optic nerve head astroglia. Vision Res. 1996;36:2015–2028. [PubMed]
Turner DL, Cepko CL. A common progenitor for neurons and glia persists in rat retina late in development. Nature. 1987;328:131–136. [PubMed]
Last Updated: November, 2013.
您正在向 biovector.net 发送关于产品 Retina Glial cells视网膜细胞/小胶质细胞/星形细胞/Muller细胞 的询问
- 公告/新闻